May 23, 2018 Download Mac OS X Lion 10.7.5 DMG free standalone setup. The Mac OS X Lion 10.7.5 DMG is an upgrade of OS X version 10.7 to improve the stability, compatibility, and security of the Mac. Mac OS X Lion 10.7.5 Review Mac OS X Lion 10.7.5 DMG is an upgrade of OS X version 10.7. https://omgability.netlify.app/mac-osx-dmg.html. Mar 16, 2016 Download Mac OS X Lion 10.7.5.DMG – Mac OS X Lion 10.7.5.ISO – OS X Lion 10.7.5 torrent – Retail DVD uploaded. Clearly, OS X Lion 10.7.x is no longer downloadable from Apple store. However, many our visitors still request this very old version for education purposes.
The Sims 4 Wicked Woohoo Mod Download has sold more than 5 million copies worldwide. You may also likeThe game has the same concept as its predecessor, The Sims 3; players control their Sims in a variety of actions and can form connections. The Sims 4 Wicked Woohoo Mod Download is the first PC game to large all-format graphs in a couple of decades. How to download wicked whims on mac. The sport has received mixed reviews since its launch.
The reaction with ethylenediamine gives: Ni(en)32+(aq) The reaction with NaOH gives: Ni(OH)2(s) The reaction with EDTA gives: NiEDTA2+(aq) Write a balanced equation for each reaction. How many d electrons are there in Ni2+? For each complex ion formed, indicate the coordination number of the copper atom. Name each complex. Do your best to. Nickel Dimethylglyoxime (Ni(dmg) 2) is one of numerous organometallic compounds manufactured by American Elements under the trade name AE Organometallics™. Organometallics are useful reagents, catalysts, and precursor materials with applications in thin film deposition, industrial chemistry, pharmaceuticals, LED manufacturing, and others.
Specification can be achieved with concentration and pH. DMG forms a chelating complex with the metal ion and forms a bright red precipitate Ni(C 4H7N2O2)2 in a slightly basic solution of 1:1 aqueous ammonia. The precipitate is washed with a 30% ethanol solution and weighed (constant weight) after drying the frits in the oven at 110 EC for 2 hours. Pour half the nickel ethylene diamine solution into a small hydrometer and add the DMG solution dropwise to form the red precipitate. Hint: Any green precipitate formation is due to Ni(OH)2 precipitate. (Add more ammonia to make it go away.) Make the ethylene diamine solution fresh if there are crystals present in the solution or if it’s more. Dimethylglyoxime, DMG (IUPAC name: 2,3-Butanedione Dioxime) (CH 3 C(NOH)C(NOH)CH 3) is a white crystalline solid that is insoluble in pure water but soluble in alcohol or a solution of NaOH, DMG has two acidic protons and thus dissolves in aqueous NaOH as a sodium salt. It is used in detection and analyzing of nickel and palladium content in. Oct 07, 2009 Hello, I would really appreciate some help. I had to do a gravimetric analysis of Nickel in Ore. My question is, how can I determine the percent by mass of the Nickel having only the mass of the Nickel complex (Ni(DMGH2) and the ore mass? I don't know how to find the mass of only the Nickel from the Nickel complex. If anyone knows, please help!!!
I had a similar issue. Mac sticky cleaner for windows.
| Names | |
|---|---|
| IUPAC name | |
Other names
| |
| Identifiers | |
| |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.201 |
| EC Number | |
PubChemCID | |
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C4H8N2O2 | |
| Molar mass | 116.120 g·mol−1 |
| Appearance | White/Off White Powder |
| Density | 1.37 g/cm3 |
| Melting point | 240 to 241 °C (464 to 466 °F; 513 to 514 K) |
| Boiling point | decomposes |
| low | |
| Structure | |
| 0 | |
| Hazards | |
| Main hazards | Toxic, Skin/Eye Irritant |
| Safety data sheet | External MSDS |
| GHS pictograms | |
| GHS Signal word | Danger |
| H228, H301 | |
| P210, P240, P241, P264, P270, P280, P301+310, P321, P330, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| Hydroxylamine salicylaldoxime | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.
Indeed, it is able to recover damaged or corrupted files and restore them into the system.Manager: OnyX contains an application manager to keep track of installed software and see how they consume system resource. It is able to increase the processing capacity for some programs and reduce those of others.Optimization: This software can clean up unnecessary registry to prevent overloading the system and causing the incessant appearance of error messages. Onyx mac download. Key FeaturesScan: OnyX carefully scans the files in the operating system and detects errors or possible failures. At the end of each analysis, it displays on the screen detected failures that may cause system damage and suggests the necessary improvements.Recovery: the main advantage with this program is that it not only detects potential bugs but also offers to correct them.
Preparation[edit]
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:[1]
Complexes[edit]
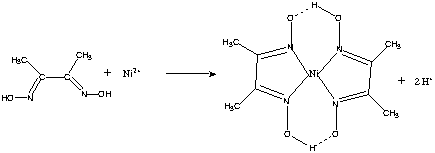
Dimethylglyoxime is used to detect and quantify nickel, which forms the bright red complex nickel bis(dimethylglyoximate) (Ni(dmgH)2). The reaction was discovered by L. A. Chugaev in 1905.[2]
Cobalt complexes have also received much attention. In chloro(pyridine)cobaloxime[3] the macrocycle [dmgH]22− mimics the macrocyclic ligand found in vitamin B12.
Ni(dmg)2 Name
References[edit]
- ^Semon, W. L.; Damerell, V. R. (1930). 'Dimethylglyoxime'. Organic Syntheses. 10: 22. doi:10.15227/orgsyn.010.0022.CS1 maint: multiple names: authors list (link)
- ^Lev Tschugaeff (1905). 'Über ein neues, empfindliches Reagens auf Nickel'. Berichte der Deutschen Chemischen Gesellschaft. 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
- ^Girolami, G. S.; Rauchfuss, T.B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual (3rd ed.). pp. 213–215.